Recently, Professor Yong Zhao’s research group has made new progress in the field of lithium-oxygen batteries. The relevant results are titled "Inhibition of Discharge Side Reaction by Promoting Solution-Mediated Oxygen Reduction Reaction with Stable Quinone in Li-O2 Batteries", and the full text is published on the International journal (ACS Applied Materials & Interfaces 2020, 12, 9, 10607-10615).
Link to the paper: https://pubs.acs.org/doi/10.1021/acsami.0c01105.
Li-O2 batteries have a theoretical energy density of up to 3500 Wh/kg. However, it still faces the challenge of poor cycle stability. One of the main reasons for the above problems is that the battery produces LiO2 intermediates during the discharge process, which can easily form a film-like discharge product Li2O2. Due to the low electronic conductivity and ion conductivity of Li2O2, the discharge reaction is terminated prematurely. In addition, the LiO2 intermediate reacts with the electrolyte easily.
Introduction of a liquid phase catalyst into the electrolyte can control the discharge reaction process of the Li-O2 battery, convert the catalytic O2 reduction reaction on the electrode surface to a liquid phase catalytic O2 reduction reaction, and delay the formation process of the film-like discharge product Li2O2, thereby increasing the discharge capacity of the battery. Among these liquid phase catalysts, quinone molecules are more special. They can not only act as an electron transfer mediator in the process of catalytic oxygen reduction, but also generate relatively stable RM-LiO2 intermediates, thereby reducing the occurrence of side reactions.
It is speculated from the discharge principle of the Li-O2 battery that these quinone molecules catalyze O2 reduction and the electrode surface catalyzes O2 reduction is a competitive process. When the reduction potential of quinone molecules is low, their ability to catalyze O2 reduction is weak, then the electrode surface catalyzes O2 reduction dominates the process, and more LiO2 intermediates are produced on the electrode surface, which not only leads to the formation of film-like Li2O2, but also causes electrode corrosion or electrolyte decomposition. On the contrary, when the reduction potential of quinone molecules is high, the possibility of O2 reduction on the electrode surface is reduced, and the possibility of O2 reduction catalyzed by the electrode surface to produce LiO2 will be reduced. However, it has been reported that the reduction potential of DBBQ, a better quinone catalyst, in the electrolyte is still low, and it is prone to decompose at a higher charge potential. Therefore, it is necessary to find a stable quinone molecular catalyst to promote the liquid-phase catalytic O2 reduction while inhibiting the electrode surface to catalyze the O2 reduction process, so as to achieve the double improvement of the lithium oxygen battery capacity and cycle stability.
In this study, benzo [1,2-b:4,5-b'] dithiophene-4,8-dione (BDTD) quinone molecules were used as liquid phase catalysts to study the effect of solution catalyzed O2 reduction and electrode surface catalyzed O2 reduction on the discharge capacity and stability of lithium oxygen battery. The half-wave potential and molecular diffusion coefficient of BDTD molecular catalytic oxygen reduction are ~2.72 vs Li+/Li and 5.05×10-7 cm2 s-1, respectively, which are higher than the corresponding parameters of DBBQ (2.67 vs Li+/Li and 2.89 × 10- 7 cm2 s-1), thus ensuring a high ORR reaction rate. In addition, the BDTD molecule has no α-H, thus showing high electrochemical stability. Therefore, the lithium oxygen battery assembled with BDTD molecules exhibits high discharge potential and capacity, as well as good cycle stability.
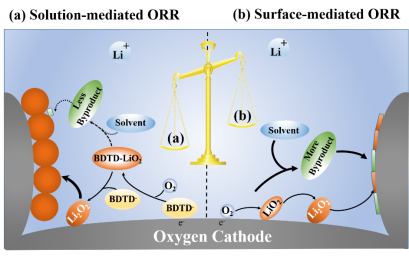
(a) Liquid phase oxygen reduction process catalyzed by BDTD molecules (due to the higher discharge potential of BDTD and the formation of a stable BDTD-LiO2 composite structure, the side reactions caused by adsorbed LiO2 are suppressed, and the capacity and cycle stability of Li-oxygen batteries are improved ); (b) Oxygen reduction process catalyzed on the electrode surface without the addition of a liquid phase catalyst (adsorbed LiO2 leads to the formation of film-like Li2O2, resulting in a decrease in battery capacity, and also causing electrode corrosion and electrolyte decomposition).
Xiao Liu, a doctoral student in the Special Functional Materials Laboratory, is the first author, and Dr. Yong Zhao is the corresponding author of the paper. This work was strongly supported by the Organization Department of the Central Committee of the Communist Party of China, National Natural Science Foundation of China, Henan Provincial Department of Science & Technology, the Henan Provincial Department of Education, and Henan University.