Recently, the research result of Prof. Gang Cheng's group "Triboelectric plasma decomposition of CO2 at room temperature driven by mechanical energy" was published in the famous international journal Nano Energy (IF=17.881, JCR First District).
Article link: https://www.sciencedirect.com/science/article/pii/S2211285521005425
While fossil fuels supply large amounts of energy for the modern society, their burning directly emits a large amount of greenhouse gases, specifically carbon dioxide (CO2), into the atmosphere, which has caused an increasingly serious environmental crisis worldwide. As one of the most important ways of solving this crisis, decomposing CO2 and converting it into high value-added chemicals have attracted widespread attention from researchers. The direct thermal decomposition of CO2 into CO is still a mainstream method for CO2 conversion. Owing to the strong thermodynamic stability of CO2, its direct thermal decomposition must be achieved at a high temperature above 2000 K, increasing its cost. Moreover, the energy consumed by high-temperature heating is mostly supplied from fossil fuels, and these processes still emit CO2, which makes it difficult for thermal decomposition to fundamentally solve the problem of CO2 emission reduction. Therefore, directly using renewable energy to efficiently convert CO2 into chemical feedstock or fuel is an ideal strategy for achieving a green and sustainable CO2 recovery.
Mechanical energy is a green and cheap renewable energy that is widely available in nature. It has many forms, including wind, tide, hydropower, and ocean wave. Triboelectric nanogenerator (TENG) is now becoming an emerging technology that converts mechanical energy into electrical energy and can efficiently collect irregular mechanical energy that widely exists in nature. TENG is characterized with high voltages, which can directly breakdown the gas and produce plasma. The high-energy electrons in plasma can interact with chemical molecules, forming highly reactive species or directly breaking chemical bonds. Therefore, plasma is endowed with an outstanding technical advantage in decomposing highly stable molecules, such as CO2 and N2. However, traditional plasma technology requires complex plasma generators and a sophisticated electrical control system. More importantly, it cannot be directly driven by renewable mechanical energy. Triboelectric plasma is directly driven by the widely distributed and effectively utilized renewable mechanical energy in nature, which is a low-cost, effective, and simple system for generating plasma over a wide range of conditions. Hence, it is highly desirable to decompose CO2 molecules through triboelectric plasma by utilizing renewable mechanical energy.
Mechanical energy directly drives CO2 decomposition to CO via triboelectric plasma.
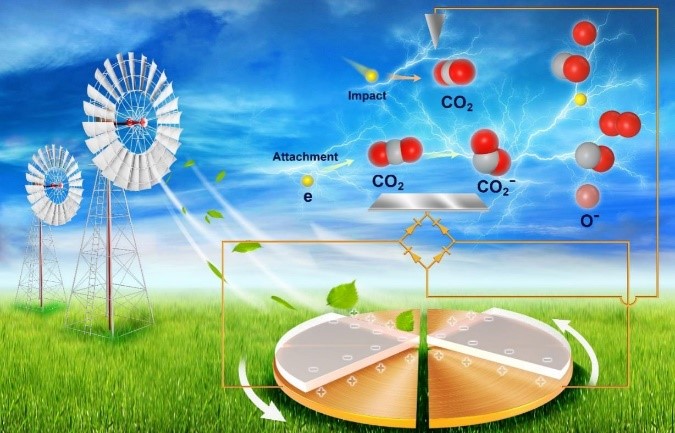
This study designed triboelectric plasma with a needle-plate discharge configuration to realize CO2 decomposition into CO with near 100% selectivity under room temperature and normal pressure conditions. Electron paramagnetic resonance (EPR) showed that highly reactive CO2− anions were produced during the decomposition process. The decomposition barrier of CO2− anions was calculated as 3.7 eV lower than that of neutral CO2 molecules, which can effectively improve the activity and energy efficiency of CO2 decomposition. The average energy of electrons in the triboelectric plasma could be reduced by regulating the needle-plate distance and corona discharge polarity, which was advantageous to the generation of highly reactive CO2− anions. The needle-plate distance (d) was 1.5 mm; thus, the CO2 decomposition to CO rate in negative corona was 2.2 μmol h−1, and an energy efficiency from eletric energy to chemical energy was 5.2%. Finally, the triboelectric plasma driven by 4.7 m s−1 wind was demonstrated to dramatically decompose CO2. This work provides a novel, sustainable, and environment-friendly strategy for converting CO2 into high value-added chemicals by utilizing mechanical energy.
Ph.D. student Sumin Li and Dr. Bao Zhang are the co-first authors of the paper, and Prof. Gang Cheng and Prof. Zuliang Du are the co-corresponding authors of this paper. This work was supported by funding from the National Natural Science Foundation of China, the Science and Technology Department of Henan Province, China Postdoctoral Science Foundation, and Henan University.